CLIA Reagents
Test Methodology:
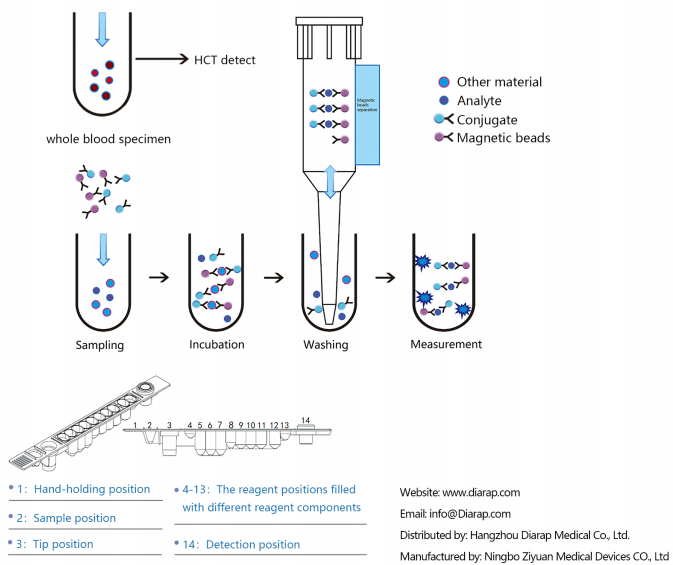
- The detecting antibody that is labeled with Alkaline phosphatase is mixed with samples and magneticparticles that are coated with capturing antibodies. After incubation, a sandwich complex is formedamong the capturing antibody on the magnetic particles, the analyte in the sample and the detectingantibody labeled with the alkaline phosphatase. The complex formed is rinsed and separated from theunreacted substances through the magnetic system of the device. Addition of the chemiluminescentsubstrate of alkaline phosphatase (AMPPD or its derivatives) to the reaction mixture to generate chemiluminescent signal (photon) at the wavelength of 470nm through the redox reaction catalyzed byalkaline phosphatase. The chemiluminescent intensity is detected by the photomultiplier tube (PT).The chemiluminescent intensity or RlU detected is proportional to the amount of the analvte in thesample, and therefore , the amount of the analvte in the sample can be determined using a calibrationcurve which is included in the Calibration Card provided in the reagent cartridge
Features:
- ·Available for Whole blood/ Serum/ Plasma
- ·14 minutes Test time for emergency items
- ·Free from cartridge Mixture, Semi-solid Magnetic bead
- ·Flexible test combination, multi-parameters in 1 run
* means CE certiied √ means China NMPA certiied
| Cardiac | Hormone | Thyroid | Vitamin | Diabetes | Inflamation | Allergy | Tumor | Anemia | Others |
| NT-PROBNP * √ | HCG* | TSH* | VD* | ISULIN * | IL-6 * √ | IGE* | AFP* | FER * | ST2* |
| CK-MB * √ | AMH* | FT4* | VB12* | CP* | HBP* | CEA* √ | PIVKA II* | ||
| MYO * √ | PRL* | FT3* | PCT * √ | NSE* √ | G-17 * | ||||
| HS -CTNI * √ | FSH* | TT4* | CRP* | PG1 * √ | |||||
| DD* √ | TESTO* | TT3* | PG2 * √ | ||||||
| BNP* | LH* | PTH * | TPSA | ||||||
| PROG* | TG* | F-PSA | |||||||
| E2* | CA199 * | ||||||||
| CA153 * | |||||||||
| CA125 * |
More items are coming … …